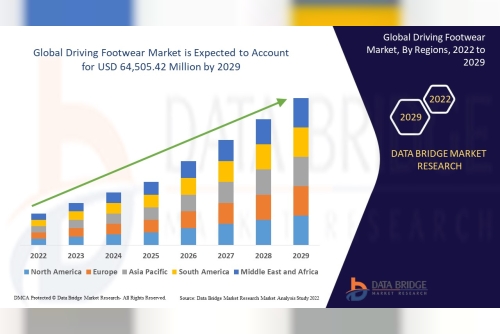
Driven by the increased adoption and rising unmet need associated with rare kidney disorders, industry stakeholders have made significant investments to enable the development of novel drug candidates
Roots Analysis is pleased to announce the publication of its recent study, titled, “Rare Kidney Disorders Market, 2022–2035”. Over 25 total pipeline candidates are available in the market across various geographical regions. Of these, 43% have been approved for the treatment of cystinosis, Fabry disease and lupus nephritis. Further, majority of the approved / under development drugs (47%) are designed for delivery via oral route.
The report features an extensive study of the current market landscape and the likely future potential associated with the rare kidney disorders market, over the next decade.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/reports/rare-kidney-diseases-market/request-sample.html
The study also features an in-depth analysis, highlighting the capabilities of various industry stakeholders engaged in this field. Amongst other elements, the report features:
§ A detailed executive summary of key insights captured during our research. It offers a high-level view on the current state of rare kidney disorders market and its likely evolution in the mid to long term.
§ A brief introduction to various aspects of rare kidney disorders. It includes a detailed discussion on the historical evolution and factors contributing to the treatment of rare kidney disorders. Further, it highlights various prominent target indications associated with rare kidney disorders, application of omics technology and its future perspectives.
§ A detailed overview of the current market landscape of more than 125 drug candidates that are either approved or currently under development for the treatment of various rare kidney disorders, based on several relevant parameters, such as phase of development (approved, registration, phase III, phase II, phase I, pre-clinical and discovery), target molecule (complement component 5 (C5), complement component 3 (C3), a proliferation-inducing ligand (APRIL), B lymphocyte stimulator (BLyS), Complement Factor B, and others), route of administration (oral, intravenous, subcutaneous, and others), and target indication (complement 3 glomerulopathy (C3G), focal segmental glomerular sclerosis (FSGS), IgA nephropathy (IgAN), lupus nephritis (LN), membranous nephropathy (MN)), type of molecule (biologics and small molecules), and type of biologic (monoclonal antibody (mAb), hormone, recombinant protein, enzyme replacement therapy (ERT), gene therapy, small interfering ribonucleic acid (siRNA) molecule and others). In addition, it presents details of the companies engaged in the development of drugs targeting rare kidney disorders, based on several relevant parameters, such as year of establishment, company size (in terms of number of employees), location of headquarters, and most active players.
§ Elaborate profiles of approved and clinical stage drugs indicated for rare kidney disorders (phase II / III or above). Each profile features an overview of the drug developer, clinical trial information related to the drug, clinical trial results, and estimated sales revenues (if available).
§ A detailed competitiveness analysis of companies engaged in the development of rare kidney disorders targeting drugs, based on their developer strength (in terms of years of experience), portfolio strength (in terms of number of drugs developed), and portfolio diversity (in terms of phase of development, target indication, and drug designation).
§ An in-depth analysis of the various collaborations and partnerships that have been inked by players engaged in the development of drugs targeting various rare kidney disorders since 2017, based on several relevant parameters, such as year of partnership, type of partnership, focus area, type of partner and regional distribution of partnerships.
§ An analysis of the funding and investments made within the domain, during the period 2016-2022, based on several relevant parameters, such as year of funding, type of funding (seed financing, venture capital financing, debt financing, grants, IPOs and other offerings), leading players (in terms of amount invested) and key investors (in terms of number of funding instances).
§ A detailed clinical trial analysis of ongoing clinical studies for the evaluation of various drug candidates targeting rare kidney disorders, based on several relevant parameters, such as trial registration year, current trial status, phase of development, key target indications, patient age, study design and key geographical regions.
§ A detailed analysis of the clinical and commercial attractiveness for drugs targeting rare kidney disorders, based on several relevant parameters, such as target patient population, dosing frequency and dose strength.
§ A case study highlighting the companies engaged in offering kidney care services, along with information on their year of establishment, company size (in terms of number of employees), location of headquarters and target indication.
The financial opportunity within the rare kidney disorders market has been analysed across the following segments:
§ Type of Molecule
§ Small Molecules
§ Biologics
§ Route of Administration
§ Oral Delivery
§ Intravenous Delivery
§ Subcutaneous Delivery
§ Other Delivery Routes
§ Target Indications
§ Atypical Hemolytic Uremic Syndrome
§ C3 glomerulopathy
§ Cystinosis
§ Dense Deposit Disease
§ distal Renal Tubular Acidosis
§ Fabry disease
§ IgA nephropathy
§ Lupus Nephritis
§ Refractory Gout
§ Key Geographical Regions
§ North America
§ Europe
§ Asia-Pacific
§ Rest of the World
For more information, please click on the following link:
https://www.rootsanalysis.com/reports/rare-kidney-diseases-market.html
You may also be interested in the following titles:
Quantum Computing in Drug Discovery Services Market : - Industry Trends and Global Forecasts, 2023-2035 Viral Clearance and Viral Testing Services Market : - Industry Trends and Global Forecasts, 2023-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800
3415
[email protected]