The global STD diagnostics market size is expected to reach USD 16.36 billion by 2030, expanding at a CAGR of 7.18% from 2024 to 2030, according to a new report by Grand View Research, Inc. The increasing disease burden of Sexually Transmitted Disease (STD) and efforts by government & non-profit organizations to increase awareness and encourage regular screenings among the younger population are the key factors augmenting the market growth. According to WHO data, around 374 million new cases of syphilis, gonorrhea, chlamydia, and trichomoniasis are recorded every year globally.
Development and commercialization of advanced products, such as next-generation HIV testing kits and multiplex PCR products, enable physicians to timely diagnose & distinguish a range of STIs and provide appropriate treatment. Seegene, Inc., a South Korea-based diagnostic company, provides a wide portfolio of Allplex, Anyplex, and Seeplex products that detect various causative agents. For instance, Allplex STI/BV Panel Assay can distinguish 28 causative agents in a single test. In April 2022, Seegene, Inc. announced the development of Allplex HPV HR Detection.
Allplex HPV HR Detection is the world’s first commercialized ‘3 Ct’ PCR assay. Seegene is focusing to apply this technology to its entire product chain, including STI, Urinary Tract Infection (UTI), and Respiratory Virus (RV) assays. This strategic technological advancement is expected to provide an additional advantage to the company that can help increase its share in the Sexually Transmitted Disease (STD) diagnostics industry.
Gather more insights about the market drivers, restrains and growth of the Global STD Diagnostics Market
The HIV National Strategic Plan is a roadmap for eliminating the human immunodeficiency virus pandemic in the U.S. by 2030. The HIV Plan, which will run from 2021 to 2025, is the country's third five-year national HIV plan in a row, with a 10-year goal of halving new human immunodeficiency virus infections by 90% by 2030. Furthermore, the Viral Hepatitis National Strategic Plan focuses on hepatitis B and C, which are sexually transmitted. In January 2021, the US Department of Health and Human Services (HHS) released a nationwide strategy to address the preventable, substantial public health threats posed by viral hepatitis in the United States. The government's beneficial actions create a favorable atmosphere for the expansion of the STD diagnostics industry.
Companies are engaging in strategic initiatives that aid in the development of new products, and regulatory approvals as well as enhance their STD diagnostics product portfolio. For instance, in August 2020, DiaSorin, Inc. received 510 (k) approval from U.S. FDA for introducing LIAISON XL MUREX anti-HBe. It is used for the diagnosis of HBV infection. This immunoassay product works on the principle of chemiluminescence.
STD Diagnostics Market Report Highlights
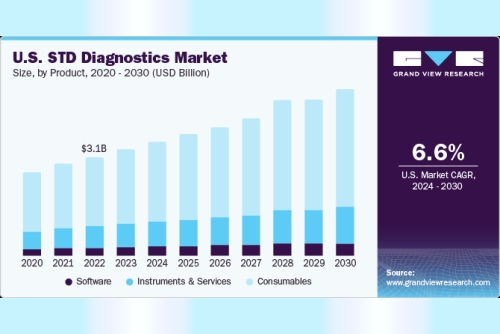
In 2023, the consumables segment accounted for the largest market share and is anticipated to maintain its position over the forecast period owing to high testing rates for the diagnosis of STDs and the commercialization of several assaysBased on technology, the molecular diagnostics segment is estimated to grow at the fastest CAGR during the forecast period owing to the increasing use of high-throughput PCR technology and the recommendation of NAAT tests for HIV testing. The development of multiplex PCR tests for the simultaneous detection of multiple pathogens is also expected to contribute to the STD diagnostics industry's growthBased on application, the HIV testing segment is expected to dominate the STD diagnostics industry in 2023, due to factors such as the high incidence rate of the disease, increasing product approvals for molecular tests, and favorable government initiativesAsia Pacific region is anticipated to experience the fastest growth over the forecast period due to an increase in testing rates for STDS, a rise in disposable income, and high disease prevalence of STD in developing countriesKey Companies & Market Share Insights
Key players are focusing on gaining market approvals for innovative products for the diagnosis of various STDs, regional & product expansions, and collaborations to maintain their share in the market.
In February 2023, Mylab introduced a series of rapid tests designed for the STD diagnostic. These tests are simple to use, can be stored at room temperature, and are suitable for use at point-of-care facilities in places with limited resources. Moreover, they find utility in blood banks for efficiently identifying transfusion-transmissible infections (TTIs) among blood donors, contributing to the reduction of transmission.In January 2023, Molbio Diagnostics launched the Truenat HSV 1/2 test for herpes simplex virus. With the ability to provide results within an hour, this product significantly boosts the company's STD diagnostic portfolio.List of Key Players in the STD Diagnostics Market
BDHoffmann-La Roche LtdHologic Inc.AbbottCepheid (Danaher)QiagenOraSure Technologies, Inc.Bio-Rad Laboratories, Inc.bioMérieux SAThermo Fisher Scientific, Inc.Seegene Inc.DiaSorin S.p.AOrder a free sample PDF of the STD Diagnostics Market Intelligence Study, published by Grand View Research.