Clinical Data Management Systems (CDMS) Market
Introduction
The Clinical Data Management Systems (CDMS) market is a critical component of the modern clinical research and healthcare ecosystem, offering sophisticated software solutions designed to collect, manage, and analyze clinical trial data with accuracy and efficiency. As the pharmaceutical, biotechnology, and medical device industries face increasing pressure to accelerate time-to-market and comply with strict regulatory standards, the demand for robust and reliable data management platforms continues to rise.
Between 2023 and 2030, the CDMS market is expected to experience significant growth, driven by the surge in clinical trials, the adoption of electronic data capture (EDC) technologies, and the integration of artificial intelligence (AI) and machine learning (ML) in clinical workflows. Furthermore, the shift toward decentralized trials, growing emphasis on real-time data analytics, and the need for seamless integration with other eClinical systems are shaping the future of the industry.
Clinical Data Management Systems Market Size
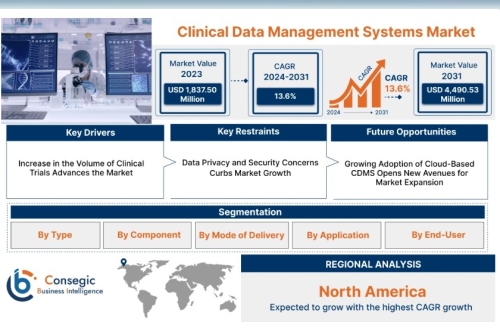
Clinical Data Management Systems Market size is growing with a CAGR of 13.6% during the forecast period (2024-2031), and the market is projected to be valued at USD 4,490.53 Million by 2031 from USD 1,837.50 Million in 2023.
Scope & Overview
The Clinical Data Management Systems (CDMS) market encompasses a wide range of software platforms and tools designed to streamline the collection, storage, validation, and analysis of clinical trial data. These systems are essential in ensuring data accuracy, regulatory compliance, and the overall integrity of clinical research processes. CDMS solutions are used extensively by pharmaceutical companies, contract research organizations (CROs), biotechnology firms, and academic institutions involved in clinical trials.
This report provides an in-depth analysis of the global CDMS market from 2023 to 2030, covering key market segments based on deployment models (on-premise, cloud-based), components (software, services), end-users (pharmaceutical companies, CROs, medical device companies, hospitals), and regions (North America, Europe, Asia-Pacific, Latin America, Middle East & Africa).
Key areas of focus include:
Market Size and Forecasts: Historical data, current market size, and future projections Technology Trends: AI integration, cloud-based platforms, and real-time analytics Regulatory Environment: Compliance with FDA, EMA, and other global regulatory bodies Competitive Landscape: Profiles of leading vendors, market share analysis, and strategic developments Growth Drivers and Challenges: Increasing clinical trial volumes, data complexity, and demand for interoperabilityThe scope of this analysis also includes emerging opportunities, such as decentralized clinical trials (DCTs), wearable data integration, and regional expansions, offering stakeholders insights into both risks and rewards in the evolving CDMS market.
Market Dynamics (DRO)
The Clinical Data Management Systems (CDMS) market is shaped by a dynamic set of factors that influence its growth trajectory and adoption across the healthcare and life sciences sectors. Understanding these Drivers, Restraints, and Opportunities (DRO) is essential to grasp the market’s evolving landscape from 2023 to 2030.
Drivers
Rising Volume of Clinical TrialsThe increasing number of clinical trials across therapeutic areas, especially for chronic diseases, rare disorders, and emerging pandemics, is a key growth driver for CDMS platforms. Adoption of Electronic Data Capture (EDC) Systems
The shift from paper-based data collection to digital platforms has boosted efficiency, data accuracy, and regulatory compliance, fueling the demand for CDMS. Regulatory Pressure and Data Compliance Requirements
Growing emphasis on Good Clinical Practice (GCP), FDA 21 CFR Part 11, and GDPR is driving the need for secure, audit-ready, and standardized data systems. Technological Advancements
Integration of AI, machine learning, and cloud-based solutions into CDMS platforms is enabling predictive analytics and real-time monitoring of trial data.
Restraints
High Implementation CostsThe initial investment for advanced CDMS solutions, including software, integration, training, and maintenance, can be prohibitive for smaller organizations. Data Privacy and Security Concerns
Handling sensitive patient data involves strict compliance with data protection laws. Any breach can have severe legal and reputational consequences. Limited Technical Expertise
Lack of trained personnel to manage, customize, and operate complex CDMS solutions can hinder adoption, especially in low-resource settings.
Opportunities
Emergence of Decentralized Clinical Trials (DCTs)The growing adoption of virtual and hybrid trials has created opportunities for flexible, cloud-based CDMS that support remote data capture and monitoring. Expansion in Emerging Markets
Increased R&D investments, regulatory reforms, and growing clinical trial activity in Asia-Pacific, Latin America, and the Middle East open new growth avenues. Integration with Other eClinical Tools
CDMS platforms that seamlessly integrate with EHRs, ePROs, CTMS, and wearable devices offer a comprehensive solution, enhancing market value and usability.
Segmental Analysis
By Type
Electronic Data Capture (EDC) Systems Widely used for real-time data entry and validation. Dominant segment due to enhanced data accuracy and audit trails. Clinical Trial Management Systems (CTMS) Used to manage operational aspects of clinical trials. Often integrated with CDMS for end-to-end trial management. Others Includes coding tools, randomization tools, eSource platforms.By Component
Software Core data management applications and platforms. Includes standalone and integrated solutions. Cloud-based software gaining traction for scalability. Services Implementation, consulting, training, and support services. Growing demand for managed services and outsourcing.By Mode of Delivery
On-Premise Offers greater control and customization. Preferred by large pharma companies with strict data security policies. Cloud-Based Rapidly growing due to lower cost, flexibility, and remote access. Enables real-time collaboration and decentralized trial support.By End-User
Pharmaceutical & Biotechnology Companies Largest end-user segment due to ongoing clinical research needs. High demand for regulatory-compliant data solutions. Contract Research Organizations (CROs) Increasingly adopting CDMS to offer competitive, tech-driven services. Prefer cloud-based platforms for scalability. Medical Device Companies Use CDMS for regulatory submissions and post-market surveillance. Hospitals & Academic Research Institutions Involved in investigator-led trials and collaborative research. Growing use of simplified or open-source CDMS solutions.By Region
North America Largest market share due to advanced healthcare infrastructure and R&D investment. Strong regulatory oversight driving adoption. Europe High clinical trial activity and GDPR compliance fueling growth. Emphasis on cross-border data sharing in EU countries. Asia-Pacific Fastest-growing region, driven by increasing trials, low-cost operations, and digital health adoption. Strong growth in China, India, South Korea, and Japan. Latin America Rising participation in global trials and supportive regulatory reforms. Middle East & Africa Emerging market with growing interest in clinical research and digital healthcare transformation.Top Key Players & Market Share Insights
The Clinical Data Management Systems market is highly competitive and dominated by several leading technology providers and contract research organizations. These companies are focusing on innovation, strategic partnerships, and global expansion to strengthen their market presence.
Oracle (USA) Medidata (USA) IBM Corporation (USA) Parexel International Corporation (USA) Clario (USA) Veeva Systems (USA) DATATRAK International, Inc. (USA) eClinical Solutions (USA) Signant Health (USA) ICON plc (Ireland)
Contact Us:
Consegic Business intelligence
Email : [email protected]
Sales : [email protected]